Single bond enthalpy in highest chloride
A definition will apppear here.
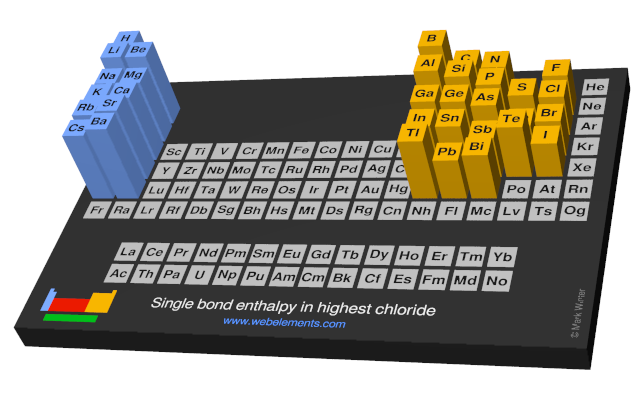

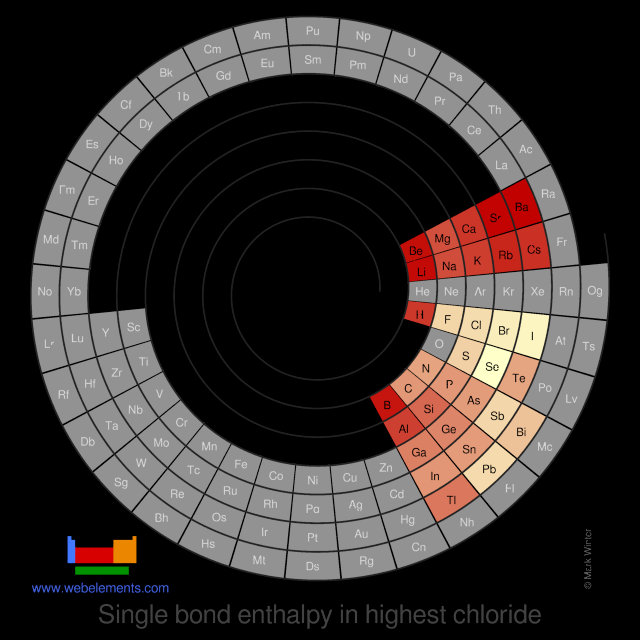
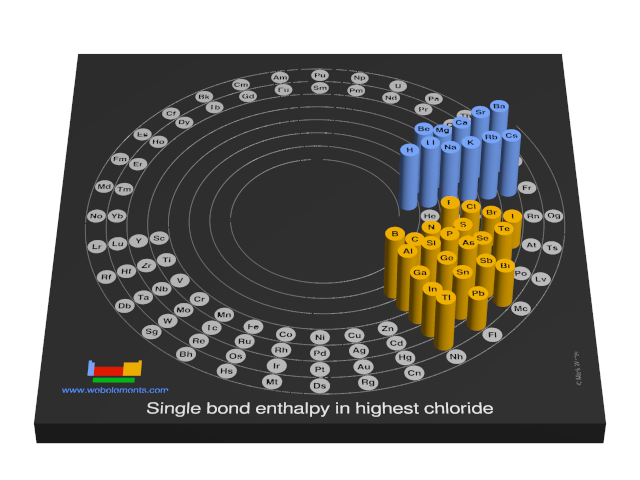

Units
kJ mol-1
Notes
None
Select from the following links to see visual periodicity representations for single element-halide single bond enthalpies in the highest halides or the single bond enthalpies for homodiatomic molecules M2.
- Single bond enthalpies in highest bromide
- Single bond enthalpies in highest chloride
- Single bond enthalpies in highest fluoride
- Single bond enthalpies in highest iodide
- Bond enthalpies (M-M single bond)
- Bond enthalpies for homodiatomic molecules M2 (not necessarily single bonds.
Each formula in the table below (M-O, M-F, and so on) is a link - select these to see visual periodicity representations for bond enthalpies involving your element of choice.
| Group 1 | Group 2 | Group 13 | Group 14 | Group 15 | Group 16 | Group 17 | Group 18 |
| M-H | M-He | ||||||
| M-Li | M-Be | M-B | M-C | M-N | M-O | M-F | M-Ne |
| M-Na | M-Mg | M-Al | M-Si | M-P | M-S | M-Cl | M-Ar |
| M-K | M-Ca | M-Ga | M-Ge | M-As | M-Se | M-Br | M-Kr |
| M-Rb | M-Sr | M-In | M-Sn | M-Sb | M-Te | M-I | M-Xe |
| M-Cs | M-Ba | M-Tl | M-Pb | M-Bi | M-Po (none) | M-At (none) | M-Rn |
| M-Fr | M-Ra | M-Nh (none) | M-Fl (none) | M-Mc (none) | M-Lv (none) | M-Ts (none) | M-Og (none) |
Literature sources
- J.E. Huheey, E.A. Keiter, and R.L. Keiter in Inorganic Chemistry : Principles of Structure and Reactivity, 4th edition, HarperCollins, New York, USA, 1993.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||