Melting point
The melting point is the temperature at which the vapour pressure of the solid and the liquid are the same and the presssure totals one atmosphere.

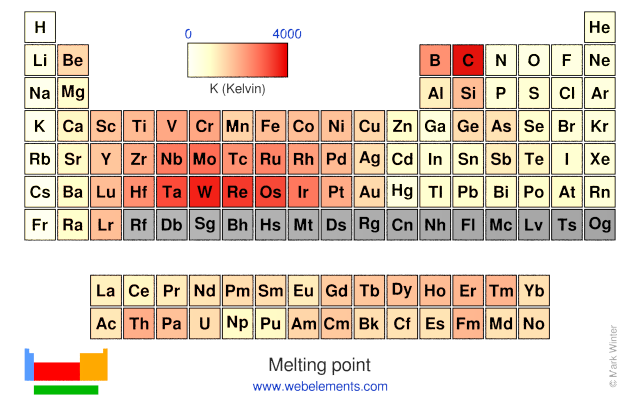



Units
K (Kelvin)
Notes
Other temperature scales include the centigrade (Celsius) scale and the Fahrenheit scale. The size of one degree is the same on the Kelvin scale (K) as on the centigrade scale (°C). The zero point is different:
temperature (K) = temperature (°C) + 273.15
Thus, the melting point of water is = 0°C = 273.15 K and the boiling point of water is = 100°C = 373.15 K
On the Fahrenheit scale (°F), the melting point of water = 32°F while the boiling point = 212°F.
Therefore the degree size is different on the Fahrenheit scale with 180 Fahrenheit degrees = 100 centigrade degrees. Conversion between centigrade and Fahrenheit is achieved using the following relationship:
temperature (°C) = [temperature (°F) -32] * 5/9
You can look at visual representations of melting points, boiling points, and the liquid range using the following links.
- Boiling point
- Boiling point of highest fluoride
- Boiling point of hydride
- Melting point
- Melting point of the highest fluoride
- Melting point of the hydride
- Liquid Range
Literature sources
- A.M. James and M.P. Lord in Macmillan's Chemical and Physical Data, Macmillan, London, UK, 1992.
- G.W.C. Kaye and T.H. Laby in Tables of physical and chemical constants, Longman, London, UK, 15th edition, 1993.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||